by Dr Quentin Welniarz, neurologist at La Pitié-Salpêtrière Hospital in Paris (Translation of the study article from the AFHA website)
The Oxytahane study
The Oxytahane study is an ongoing clinical trial exploring whether high-flow oxygen can stop disabling movement attacks in people with Alternating Hemiplegia of Childhood (AHC). Earlier observations suggested that giving oxygen at the start of an attack may help relieve symptoms more quickly. This study formally tests that approach by comparing oxygen with a placebo treatment during attacks over a five-week period. Researchers will assess how often attacks stop within 30 minutes of treatment. The trial is currently underway, and it is hoped that results can be shared at the 14th ATP1A3 Symposium.
The AHC-Oxytahane Study is led by Dr. Eleni Panagiotakaki and organised by a medical team in Paris including Prof. Flamand-Roze, Dr. Quentin Welniarz and Dr. Clément Desjardins. It has been co-funded by seven AHC organisations: Iceland, Netherlands/Belgium, UK, France, Cure AHC, AHC Foundation, and Hope for Annabel.

Dr Quentin Welniarz is a research engineer at the Brain Institute at La Pitié-Salpêtrière Hospital in Paris, and he is working with Prof. Emmanuel Flamand-Roze on the AHC-O2 study (or the Oxytahane study for the AP-HP).
Reminder about the disease:
Alternating Hemiplegia of Childhood Pathogenic variant of the ATP1A3 gene in 80% of cases.
Clinical aspects
- Variable degree of intellectual disability
- Hypotonia
- Motor disorders
- Epileptic seizures
- Non-epileptic seizures: plegic or dystonic
Review of seizure treatments and existing medications:
There are 2 types of treatment:
- Crisis treatments:
- Benzodiazepines (retrospective studies)
- Benefits: also works for epilepsy, effective (sleep)
- Drawbacks: sedation, swallowing disorders, respiratory problems
- Other sedatives: melatonin, phenobarbital
- Benzodiazepines (retrospective studies)
- Preventive (long-term) treatment:
- Flunarizine: many retrospective studies, one randomised controlled trial with 12 patients, negative result.
- Note: Flunarizine is used off-label in routine practice because some uncontrolled studies showed benefit. It is no longer manufactured in France but is available in Spain and Belgium. A specific protocol for Alternating Hemiplegia was set up with help from the ANSM.
- Triheptanoin: a randomised controlled trial with 10 patients, negative result
- Others (retrospective studies or case series): ketogenic diet, Topiramate, steroids, Amantadine, Memantine, Aripiprazole, ATP, Coenzyme Q, Acetazolamide…
- Flunarizine: many retrospective studies, one randomised controlled trial with 12 patients, negative result.
We are left helpless, because we lack effective treatments for AHC; to date, no treatment has shown efficacy in a randomised controlled trial.
Alternating Hemiplegia of Childhood: towards a new treatment?
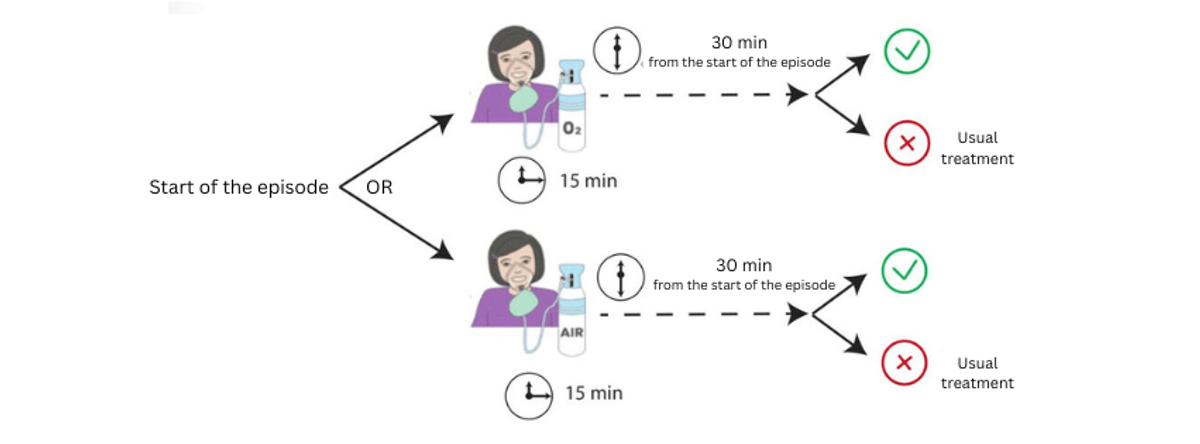
Procedure: 100% high-flow oxygen (12 L/min) via a high-concentration mask (with reservoir), given as quickly as possible after the start of a crisis and for 15 minutes.
History: After observing one patient who responded quite dramatically to inhalation of neopad laughing gas (composed of 50% nitrous oxide + 50% oxygen), the idea was explored of giving oxygen alone.
A first trial in one child with AHC showed that a large majority of episodes stopped prematurely in less than 15 minutes. This was the subject of a first publication (https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/mds.29357).
A second trial in two children with both plegic and dystonic episodes was the subject of a second publication (https://movementdisorders.onlinelibrary.wiley.com/doi/10.1002/mds.29561).
It is important to explain that the O₂ dosage protocol has been known for several years to treat severe migraines in adults and children.
It is expected that the effect will be concrete only on motor episodes (this excludes episodes with epileptic activity). It should also be noted that the statistics are only indicative because the study involves few patients.
| Oxygen efficiency: % of episodes resolved | Patient 1 | Patient 2 | Total |
|---|---|---|---|
| Dystonic episodes | 97% | 60% | 92% |
| Plegic episodes | 100% | – | 100% |
| All episodes | 98% | 70% | 93% |
| Severe episodes | 97% | 0% | 92% |
OXYTAHANE (OXYgen Acute Treatment for Alternating Hemiplegia of Childhood)
Main Objective
To evaluate, in patients with Alternating Hemiplegia of Childhood (AHC), the effect of high-flow oxygen inhalation (versus placebo) used as an acute treatment of motor episodes (dystonic or plegic) on the proportion of motor episodes resolved 30 minutes after symptom onset over 5 weeks.
Experimental Design
Randomised, placebo-controlled, cross-over trial, double-blind, consisting of two successive 5-week periods, conducted at two centres (Paris and Lyon).
Participants
24 patients (minors and adults) with AHC and ATP1A3 mutation, averaging at least one dystonic or plegic crisis per week of at least 30 minutes.
Inclusion Criteria
- Age > 1 year (no upper age limit)
- Ability for the patient to perform the intervention (high-flow oxygen via mask), either alone or with help
- AHC with ATP1A3 mutation and at least one qualifying crisis per week
- No background treatment or stable background treatment for the past month that can be maintained throughout the study
- Free, informed, signed consent
- Affiliation with or beneficiary of a social security scheme (except AME)
Exclusion Criteria
- Inability by patient or caregiver to complete evaluations (unable to read or understand scales, no access to smartphone or computer)
- Severe uncontrolled chronic respiratory disease (asthma, COPD, obesity, neuromuscular disease), acute systemic pathology, or congenital heart disease for which oxygen therapy poses risk
- Pregnant or breastfeeding
- Participation in another interventional health product trial
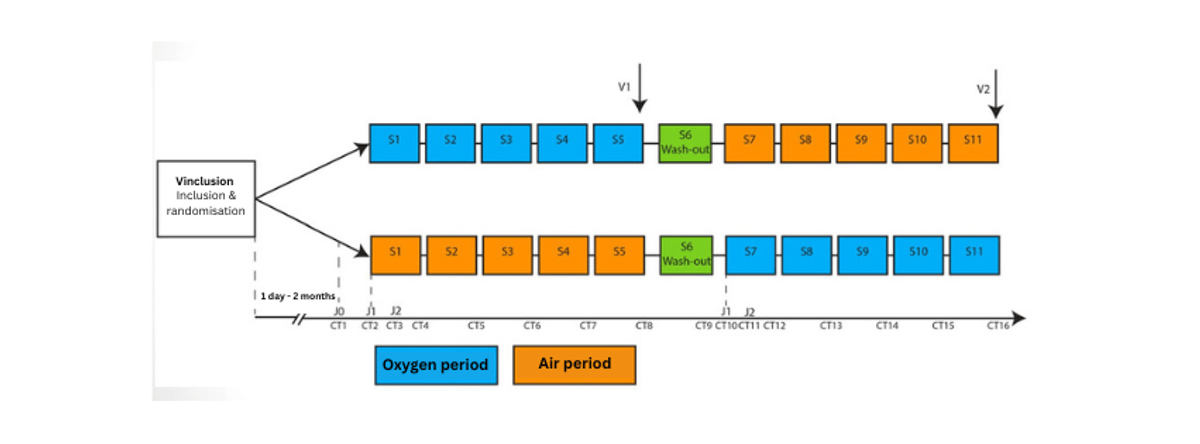
Study procedure
The washout period is a one-week break.
Note: no one knows who is in which group so as not to influence the interpretation of the results.
Study Procedures:
- Test treatment: High-flow oxygen (12 L/min) via high-flow mask with reservoir for 15 minutes, started as soon as possible after crisis onset
- Reference treatment: High-flow medical air (12 L/min) via high-flow mask with reservoir for 15 minutes, started as soon as possible after crisis onset
- Usual crisis treatments (benzodiazepines such as Buccolam, Rivotril, or Valium, melatonin) are allowed if the crisis has not resolved 30 minutes after onset.
Primary Endpoint
Proportion of motor episodes (dystonic and/or plegic) resolved 30 minutes after symptom onset during each 5-week period.
Why to Participate?
Positive points:
- Allows formal validation of an acute treatment in AHC
- Participate in a promising human endeavour!
Negative points:
- Travel required for the inclusion visit (other visits are remote)
- Requirement to complete the electronic patient diary for 11 weeks
Electronic Patient Diary
At each episode, families will log into the electronic diary using a personal anonymous ID and password to protect health data. The diary presents a series of questions, some with free-text responses, submitted via phone or computer. Responses are analysed by statisticians.
To be completed by families
- There are 11 questions
- It is easy to use, but it may still be a burden for families as they will need to fill in the application form accurately
- Send responses
- Responses analysed by statisticians
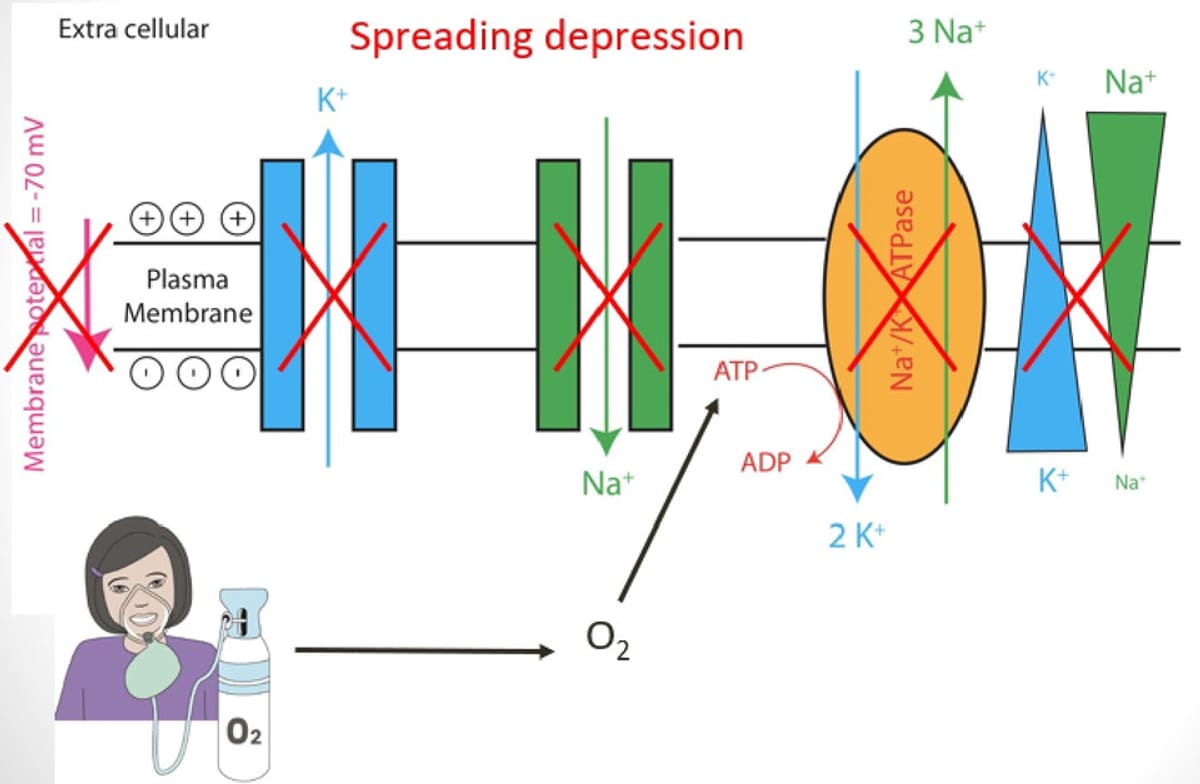
Mechanistic Hypotheses
- The effect of oxygen could be related to cortical spreading depression (SD)
- SD is associated with neuronal dysfunction and other paroxysmal abnormal movements (paroxysmal kinesigenic dyskinesia, hemiplegic migraine)
- O₂ inhalation boosts ATP to ADP conversion and promotes proper sodium-potassium pump function.
The medical research teams are fully committed to ensuring the success of this project and relieving children.
